1. Introduction
Isocitrate dehydrogenase (IDH) mutations are among the most frequent genetic alterations in acute myeloid leukemia (AML) - they are detected in approximately 20% of patients. While ivosidenib and enasidenib have been approved as targeted therapy for IDH1 and IDH2-mutated AML, respectively, relative outcomes between the IDHmut isoforms remain unclear across various therapeutic options. The aim of this study was to compare response and survival between IDH-mutated cohorts of AML when treated with intensive chemotherapy, venetoclax-based strategies, or targeted therapies.
2. Methods
We analyzed 93 patients with newly diagnosed or relapsed or refractory IDHmut AML from January 1, 2013 to April 18, 2023 at VCU Massey Comprehensive Cancer Center. We recorded baseline patient-related and disease characteristics, including dates of regimen initiation and survival. Categorical comparisons used Fischer's exact test. We excluded those with cooperating IDH1 and IDH2 mutations. We analyzed survival by the Kaplan-Meier method with significance determined by the log-rank test. The event for calculating the overall survival (OS) was the date of death. Patients were otherwise censored at the date of last contact.
3. Results
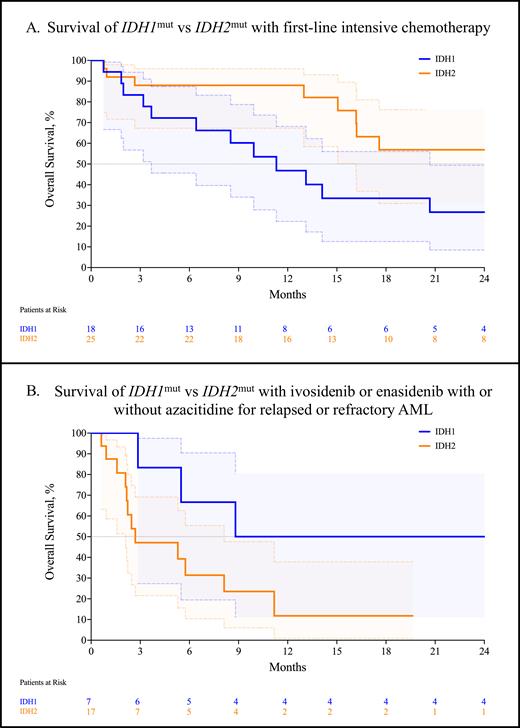
We analyzed 43 patients with IDH1mut or IDH2mut AML treated with first-line intensive chemotherapy with conventional 7+3, CPX-351, or FLAG-IDA with or without venetoclax; we divided the patients into two cohorts: IDH1 and IDH2. We noted no significant differences in the presence of cooperating NPM1 mutations (25.0% vs 37.0%, p = 0.529) or in the proportion of ELN 2022 adverse-risk disease (45.0% vs 40.7%, p > 0.999). The composite complete remission (CRc; CR + CRi + CRh) rate for IDH1mut AML was 66.7% (95% CI, 43.7-83.7) compared to 60.0% (95% CI, 40.7-76.6, p = 0.755) for IDH2mut. The rate of MRD negativity was 50.0% (95% CI, 18.8-81.2) for IDH1mut and 30.0% for IDH2mut (95% CI, 10.8-60.3, p = 0.607). Strikingly, the median overall survival significantly favored the IDH2mut cohort at 25.5 months vs 11.3 months for IDH1mut (p = 0.047, Figure A).
Next, we analyzed the outcomes of 19 patients with IDHmut AML treated with first-line venetoclax and a hypomethylating agent (VEN+HMA). We noted a lower proportion of cooperating NPM1 mutations in the IDH2mut cohort compared with the IDH1mut cohort (36.4% vs 75.0%, p = 0.170). Despite this, the CRc rate favored the IDH2mut cohort compared with the IDH1mut cohort (62.5%; 95% CI, 40.7-76.6, vs 20.0%; 95% CI, 30.6-86.3, p = 0.266). We noted no significant difference in overall survival between the IDH1mut or IDH2mut cohorts treated with HMA+VEN (12.2 months vs 14.8 months, p = 0.834).
In the relapsed or refractory setting, we analyzed 17 patients that received either ivosidenib or enasidenib with or without azacitidine. The CRc for the ivosidenib cohort was 33.3% (95% CI, 5.9-70.0), compared with 28.6% (95% CI, 5.1-64.1, p > 0.999) for the enasidenib cohort. The median overall survival favored the ivosidenib cohort at 18.8 months compared to 2.7 months for the enasidenib cohort, which approached statistical significance (p = 0.069).
4. Discussion
The median overall survival of IDH1mut AML is significantly worse compared to IDH2mut AML when treated with intensive chemotherapy. The significance of the survival disparity disappears with patients treated with HMA+VEN, suggesting both isoforms of IDHmut AML benefit from venetoclax. Investigators should be cautious when combining IDHmut AML cohorts while reporting the results of clinical trials in molecularly selected patients.
Disclosures
Grant:Prescient Therapeutics: Research Funding. Maher:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Sobi (Doptelet): Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal